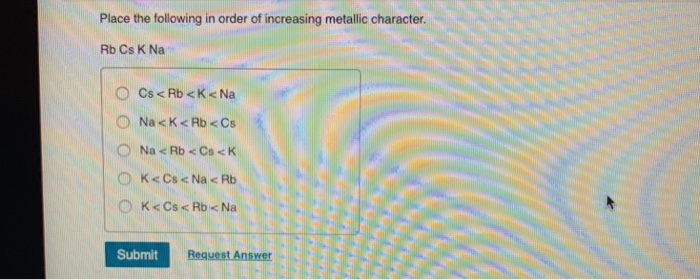
Place the Following in Order of Increasing Metallic Character.
Place the following in order of increasing metallic character. Rb K Ca CORRECT Place the following in order of decreasing metallic character.

Periodic Trends Chemwiki Electron Affinity Ionization Energy Chemistry Textbook
Place the following in order of decreasing metallic character.
. E 58 Place the following in order of increasing metallic character. Alkali metals are the most electropositive elements and have maximum metallic elements. C An orbital that penetrates into the region occupied by core electrons.
Describe the reaction of the noble gases with metals. So its going to be a pro mean germanium gallium and then calcium. K Cs Rb Na.
Na K Rb Cs. P As K A P As K B As P K C K P As D As K P E K As P Answer. The increasing order of the metallic character is.
Place the following in order of increasing IE1. Is more shielded from nuclear charge than an orbital that does not penetrate and. K Cs Na Rb.
Cs Rb K Na. What are the phase of matter. To rank items as equivalent overlap them.
K As PCORRECT Give the ground state electron configuration for Rb. Metals are the ones which has the tendency to lose the electrons and are electropositive in nature. E None of the above is true.
57 Place the following in order of decreasing metallic character. G e G a M g C a K. Na P Al Ar.
This is the best answer based on feedback and ratings. What did Rutherford do in 1911. B Na Mg Al Si Cl.
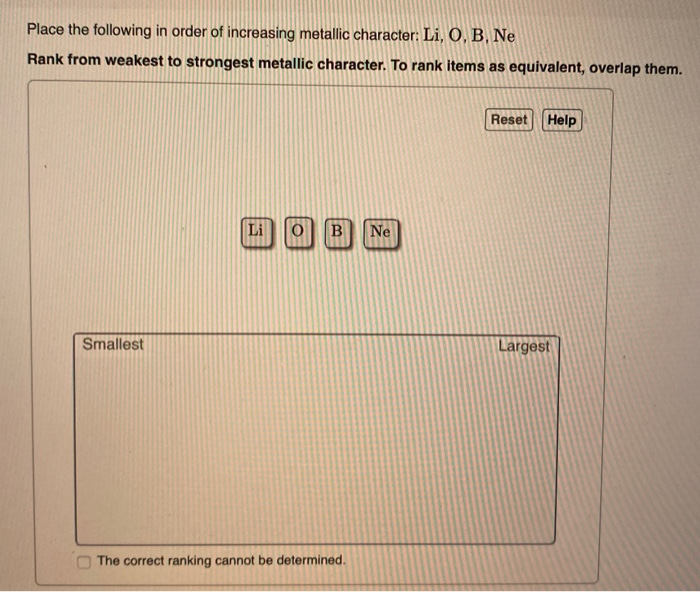
Li O B Ne Rank from weakest to strongest metallic character. Place the following in order of increasing metallic character. P As K A P As K B As P K C K P As D As K P E K As P Answer.
Cl Si Al Mg Na. E 58 Place the following in order of increasing metallic character. Of the questioner wants this place the following in order of decreasing metallic character.
Na Mg Al Si Cl. A An orbital that penetrates into the region. The metallic character of an element is defined as the easiness of its atom is losing electrons.
Fill in each of the following blanks using higher or lower more or less. Rb Cs K Na. Rb Cs K Na.
So its going to be a pro mean germanium gallium and then calcium. Arrange the following elements in the order of their decreasing metallic character Na Si Cl Mg Al. The term metallic character refers to a set of chemical characteristics that are linked with metals.
Reset Help Li O B Ne Smallest Largest The correct ranking cannot be determined. Which describes the valance electrons of magnesium. P As K P As K As P K As K P K As P K P As.
Rb Cs K Na. Na K Rb Cs. So we have magnesium phosphorus um aluminum and then are gone.
So were between bro mean Really um um calcium and then gallium. Metallic Character _________ going left to right through period 3. The majority of metals are malleable and ductile meaning they can be deformed without breaking.
This happens due to the fact that while moving from left to right in a period the number of electrons and protons in an atom increases and this. The question here asked us to place the following in order of increasing metallic character. K Ca Rb Ca K Rb K Ca Rb Rb Ca K Rb K Ca Ca Rb K.
Join our Discord to connect with other students 247 any time night or day. As we move from left to right in periodic table metallic characters decreases and non-metallic characters increases. According to the modern periodic table the metallic character of an element decreases while moving from left to right across a period.
Fr Sb In S Ba Se. Were always here. D 57 Place the following in order of decreasing metallic character.
Sr has a _______ ionization energy and is __________ metallic than Sb. K As P. So were between bro mean Really um um calcium and then gallium.
Na Rb Cs K. Solution for Place the following in order of increasing metallic characterNa P Al Ar. So in this particular case its going to.
Of the questioner wants this place the following in order of decreasing metallic character. Place the following in order of increasing metallic character. Place the following in order of increasing metallic character.
Place the following in order of increasing metallic character. Al Na Si Ca Mg. Arrange these elements in order of increasing metallic character.
Na K Rb Cs. Therefore has a lower energy. Na Al Mg Cl Si.
Na Al P Ar. D Two electrons in the same orbital can have the same spin. Which species has the highest ionization energy.

Metallic And Non Metallic Character Periodic Trends Examples Videos

Increasing Order Of Metallic Character Will Be Youtube
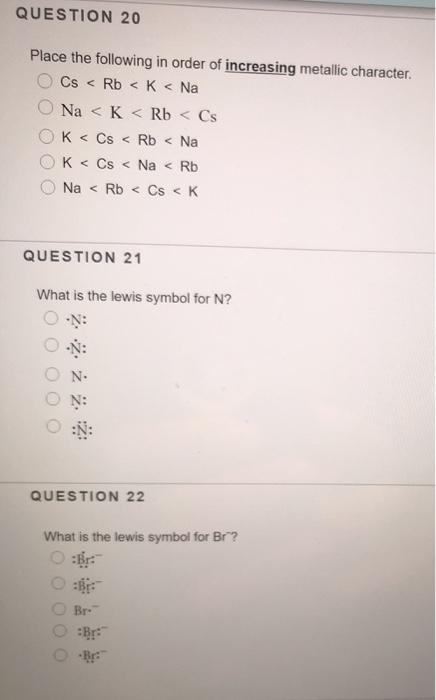
Solved Question 20 Place The Following In Order Of Chegg Com
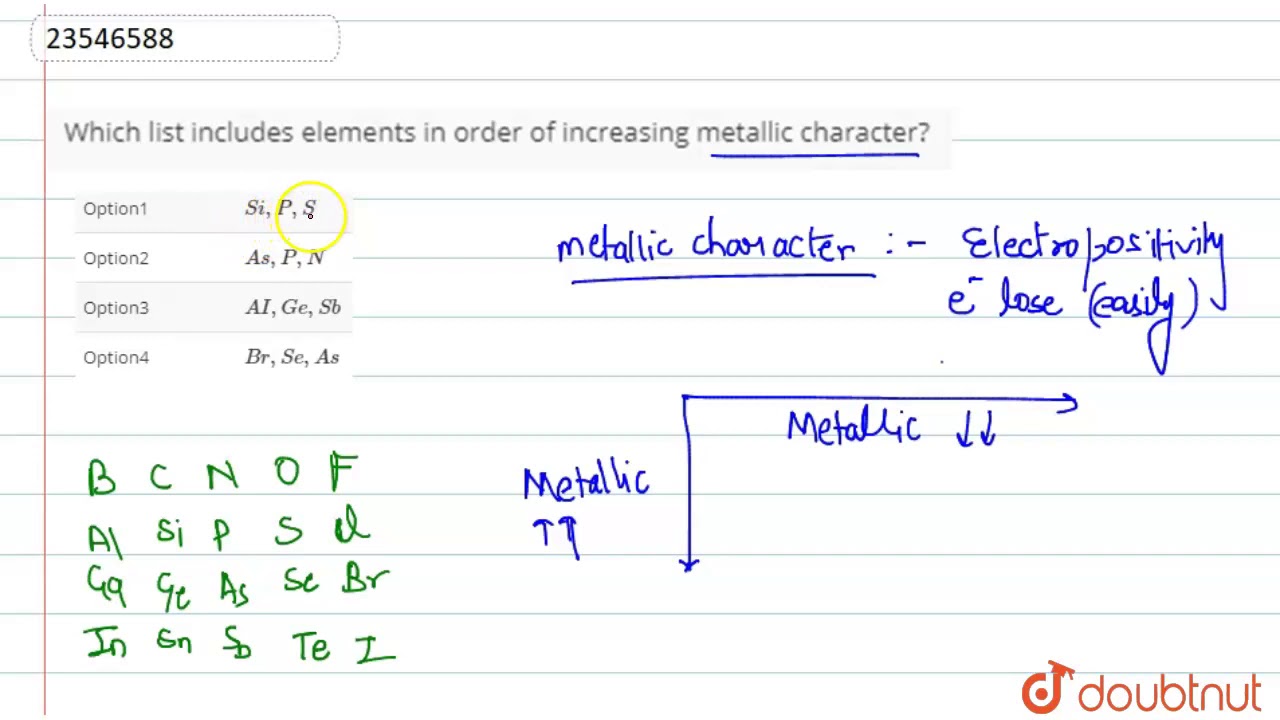
Which List Includes Elements In Order Of Increasing Metallic Character Youtube

Solved Place The Following In Order Of Increasing Metallic Chegg Com

Metallic Character Of Transition Metals Transition Element

Metallic And Non Metallic Character Periodic Trends Examples Videos

Metallic And Nonmetallic Character Chemistry For Non Majors
Metallic Character The Periodic Table Of Elements

Periodic Trends Metallic And Nonmetallic Character Ck 12 Foundation

Metallic Character Trend On The Periodic Table

Solved Place The Following In Order Of Increasing Metallic Chegg Com

How Do You Arrange Elements In Order Of Increasing Metallic Character
Solved Place The Following In Order Of Increasing Metallic Chegg Com
Solved How Do We Determine What The Metallic Character Is Course Hero
Solved Place The Following In Order Of Increasing Metallic Chegg Com

Arrange The Following In Increasing Order Of Metallic Character Cs Na Li K Rb
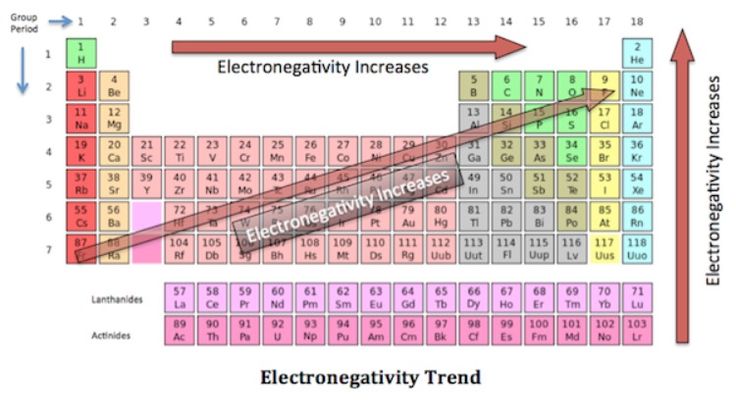
Metallic Nonmetallic Property Acidic Basic Property In The Periodic Table Science Online

Comments
Post a Comment